Article Text
Statistics from Altmetric.com
Introduction
Helicobacter pylori is a slowly growing, gram negative microaerophilic bacterium that colonises the gastric mucosa. Cross-sectional epidemiological studies from both developed and developing countries suggest that the prevalence of H. pylori infection worldwide is declining, and may even be less than 10% in ‘westernised’ countries.1 ,2 H. pylori infection is acquired during the first decade of life and infection usually persists without treatment. Spontaneous clearance has been reported, although co-incidental antibiotic exposure may influence such ‘clearance’. H. pylori is the causative agent for diseases including peptic ulcer disease (PUD), chronic gastritis, gastric mucosa associated lymphoid tissue lymphoma and is the single-most important risk factor for developing gastric cancer.3 ,4 Putative extraintestinal associations have included refractory iron deficient anaemia, short stature and idiopathic thrombocytopenic purpura, although data supporting these associations in children must be interpreted with caution.4 ,5 The majority of children infected with H. pylori are asymptomatic. Following successful treatment, the risk of true reinfection in childhood is extremely low, at least in developed countries.6 Recrudescence describes re-colonisation with the same strain within 12 months, while re-infection refers to colonisation with a new strain, more than 12 months after eradication. Clinicians must carefully bear in mind these salient aspects of the pathogenesis and natural history of H. pylori-associated diseases as they consider whether testing their patients for H. pylori is indicated.
Investigations for H. pylori
The primary indication for investigation in children remains to diagnose the cause of significant symptoms and not simply to detect the presence of H. pylori. Testing for H. pylori is not helpful unless it alters clinical management, and consideration of the age-related pre-test probability of particular diseases (rather than infection alone) is essential. There remain certain scenarios in which screening for the presence of H. pylori is not absolutely indicated but may be considered by clinicians (see box 1). As clinical and epidemiological data on the manifestations of H. pylori improves over time, remaining ambiguity should diminish. Box 1 summarises the current recommended indications for testing developed by the recent joint North American Society for Pediatric Gastroenterology, Hepatology and Nutrition/European Society for Paediatric Gastroenterology, Hepatology and Nutrition (NASPGHAN/ESPGHAN) consensus conference.7 Ideal tests are non-invasive, highly accurate, inexpensive, and readily available. An ideal test for H. pylori should also differentiate between active and past infection and discriminate between simple H. pylori infection and H. pylori—associated disease. No such ideal test currently exists. Invasive (requiring endoscopy) and non-invasive tests are available to diagnose H. pylori infection and clinicians must consider their suitability and validity for use in children and the likely implications of a positive or negative result before they are requested (tables 1 and 2).
Summary of ESPGHAN/NASPGHAN evidence-based consensus guidelines for H. pylori infection in children.7 (Adapted from Journal of Pediatric Gastroenterology and Nutrition, Wolters Kluwer Health publishers, with permission)
-
Clinical investigation of gastrointestinal symptoms should aim to determine their underlying cause and not merely the presence of H. pylori infection.
-
Diagnostic testing for H. pylori infection is not indicated in children with functional abdominal pain.
-
Testing for H. pylori may be considered in the following circumstances:
-
those with first degree relatives with gastric cancer.
-
those with refractory iron deficiency anaemia, where other causes have been ruled out.
-
-
There is currently insufficient evidence supporting testing in the following conditions:
-
otitis media, upper respiratory tract infections, periodontal disease, food allergy, idiopathic thrombocytopenic purpura or short stature.
-
-
‘Test and treat’ strategies are not recommended in children.
-
The initial diagnosis of H. pylori infection should be either based on a positive biopsy culture or based on positive histopathology (antrum and corpus biopsies) plus a positive rapid urease test.
-
Both urea breath tests and ELISA-based H. pylori stool antigen tests are reliable noninvasive tests for determining H. pylori eradication.
-
Tests based on the detection of H. pylori antibodies (IgG, IgA) in serum, whole blood, urine and saliva are not reliable for use in the clinical setting.
-
Initial testing for H. pylori should wait a minimum of 2 weeks after stopping PPI therapy and 4 weeks after stopping antibiotics.
-
H. pylori eradication is recommended in H. pylori-positive peptic ulcer disease.
-
H. pylori treatment may be considered in the following situations:
-
biopsy-proven infection in the absence of peptic ulcer disease
-
H. pylori infected children whose first degree relative has gastric cancer.
-
-
Reliable non-invasive tests for eradication are recommended no sooner than 4 weeks following completion of therapy.
ESPGHAN/NASPGHAN, North American Society for Pediatric Gastroenterology, Hepatology and Nutrition/European Society for Paediatric Gastroenterology, Hepatology and Nutrition.
Tests for H. pylori and Helicobacter-related disorders
Comparison of positive and negative predictive values of non-invasive H. pylori tests
Standardised test methodology is also necessary to obtain reliable, comparable results. Any local changes in methodology or lack of local validation could have a strong negative impact on the reliability of the test.8
Invasive tests
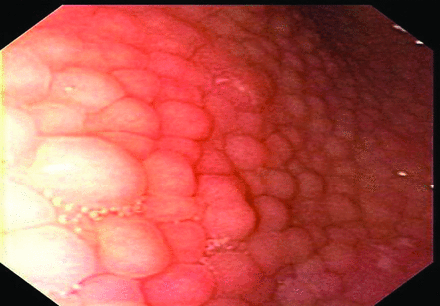
The reference standard for investigating H. pylori infection and its consequences in childhood remains combining upper gastrointestinal endoscopy with accompanying biopsies for histology and microbial detection and/or culture.11 Endoscopy allows identification of macroscopic stigmata of oesophago-gastro-duodenal disease. Gastric antral nodularity is seen more frequently in children than in adults and while associated with, it is not specific for H. pylori gastritis (figure 1).12 Two broad patterns of H. pylori gastritis have been described—antral predominant gastritis, (linked to increased peptic ulcer risk) and pangastritis (linked to increased risks of gastric atrophy). Therefore, biopsies of the gastric antrum and body should be taken at endoscopy. Biopsy specimens obtained in the pre-pyloric antrum have the highest yield for detecting H. pylori infection. Children with endoscopically documented peptic ulcer disease should have multi-site biopsies.
Gastric antral nodularity. Nodules measure up to 5 mm in diameter, have a smooth surface and are the same colour as the surrounding mucosa. It is frequently referred to as antral nodular gastritis.
Histopathology
H. pylori associated inflammation results in a superficial infiltrate with substantial numbers of plasma cells and lymphocytes within the gastric mucosa on H&E staining.13 In children, H&E and Giemsa stains have a sensitivity of 82% and specificity of 95% (figure 2).14 The site from which a biopsy is taken affects the accuracy of diagnosis, and in children the optimal biopsy location for detecting H. pylori histologically is the mid-antrum at the lesser curvature.15 Inter-observer variability in assessment of lesions histologically highlights the importance of biopsy interpretation by an experienced pathologist and clinical correlation with paediatric gastroenterologists.
Histopathological appearances of H. pylori associated gastritis: (A) prominent gastric antrum lymphoid follicle in a patient with antral nodularity and H. pylori infection (H&E stain). (B) multiple Helicobacter-like organisms seen in gastric antrum biopsy (arrows: Toludene Blue stain).
Biopsy culture
Biopsy culture from gastric biopsies is a tedious but reliable procedure with a high degree of sensitivity when performed carefully; in practice, however, many laboratories without extensive experience of H. pylori culture have low rates of successful culture. Close liaison with experienced microbiology laboratories is therefore essential to ensure that culture sampling, culture techniques and results are reliable, valid and audited. Culture also enables screening of antibiotic sensitivity, which guides therapeutic management. Both phenotypic and genotypic methods are available for antimicrobial susceptibility testing.
Rapid urease test
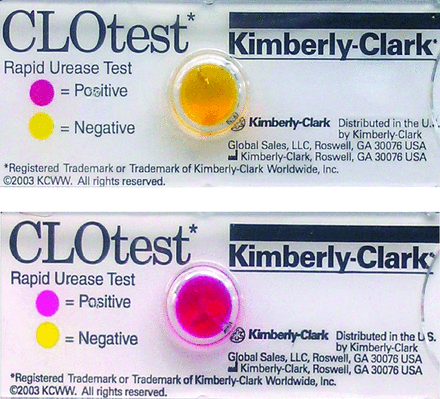
The urease activity of H. pylori catalyses the hydrolysis of urea into ammonia and carbon dioxide. The production of ammonia leads to an increase in the local pH. Biopsies are placed within a gel containing urea and a pH indicator and a colour change occurs as urea is broken down by the bacteria (figure 3).11 Rapid urease tests (RUTs) have been validated for use in paediatrics, although they are limited by a significantly lower sensitivity compared with that of histology—possibly related to a lower mucosal bacterial load.16 New RUTs are emerging, but as with all RUTs, their interpretation is influenced by the number of tissue specimens tested, the location of biopsy sites, bacterial load and previous use of antibiotics or proton pump inhibitors.
An example of a negative (top; yellow) and positive (bottom; pink) rapid urease test.
Endoscopy is not without its disadvantages, including procedural risk, anaesthetic/sedation requirements, relative expense, and limited access to appropriate paediatric specialist centres. This reinforces the importance of having a clear and valid clinical indication to perform an endoscopy in children.
Non-invasive tests
While endoscopy with histopathology remains the standard of care to investigate consequences of H. pylori infection, non-invasive tests are well performing, validated post-treatment tests in children.
Urea breath tests
The urea breath test (UBT) is the most commonly used non-invasive test for H. pylori infection in children having a high sensitivity and specificity (>95% each), although it has some limitations in children aged under five. Urea (50–100 mg) labelled with the nonradioactive isotope 13C is ingested with a test meal (to delay gastric emptying). Breath samples are collected at variable times post-ingestion.17 For optimal results, the gastric environment should be acidic (eg, giving citric acid pre-test).18 Detection requires a mass spectrometer and results are reported as delta over baseline (DOB) values for the measured ratio 13CO2/12CO2. DOB values exceeding a fixed cut-off value are considered indicative of H. pylori infection.19
Stool antigen tests
Stool testing for H. pylori antigen is an inexpensive, non-invasive method of determining H. pylori infection. Most laboratory stool antigen tests are immunosorbent assay-based tests that use microplates coated with antibodies to detect H. pylori antigens. Stools can be obtained at any convenient time and even stored frozen until processed. When comparing the cost-effectiveness of this method against UBT, the stool test requires an optical spectrophotometer (usually present in most laboratories), has negligible maintenance costs and does not require dedicated personnel.17
Indications and limitations
In children with recurring abdominal pain, should a non-invasive test for H. pylori be obtained first to avoid endoscopy?
The primary aim of clinical investigation of gastrointestinal symptoms is to determine the underlying cause of the symptoms, and not solely the presence of H. pylori infection. Presenting complaints like pain, nausea, or reflux symptoms are nonspecific and may be caused by diverse conditions within or outside the digestive tract. Such differential diagnoses may be overlooked if a non-invasive test for H. pylori infection is positive and treatment commenced. When comparing endoscopy and non-invasive tests, it must be remembered that non-invasive tests screen for H pylori infection only. Endoscopy will explore differential diagnoses such as gastro-oesophageal reflux disease, eosinophilic oesophagitis and peptic ulcer disease, or indeed confirm normal findings. For example, a number of studies have confirmed the lack of evidence for a causal relationship between H. pylori infection and non-specific (functional) recurrent abdominal pain.20 ,21 Data from recent European multicentre studies of paediatric endoscopy suggest a relatively low prevalence of pathology at endoscopy, with one study reporting peptic ulceration in <5% of children aged under 8 years and <10% of adolescents who had confirmed H. pylori infection and were symptomatic at endoscopy.3 ,22 Consensus expert opinion, based on moderate strength of evidence, does not recommend detection of H. pylori infection by a non-invasive test followed by empiric treatment of positive test—a ‘test and treat’ strategy—because the focus of investigations should be to determine the cause underlying symptoms. Adult guidelines currently differ in this respect (table 3).23
-
Clinical message:
-
There is inadequate evidence to support a causal relationship between H. pylori gastritis and abdominal pain in the absence of ulcer disease.
-
Patients with abdominal pain should not be non-invasively tested for H. pylori infection, unless endoscopy is being pursued in search of organic disease.
-
Screening for H. pylori in the general paediatric population is not recommended.
-
Current evidence does not support an empiric ‘test and treat’ strategy in children.
Differences in management guidelines between children and adults with H. pylori infection
Following eradication therapy in children with H. pylori—associated ulcer disease, is a repeat endoscopy and biopsy needed to confirm eradication?
Studies suggest that children with PUD have a higher rate of relapse without full clearance of H. pylori infection. Eradication of H. pylori in adult patients with PUD has been shown to significantly reduce the rate of relapse for ulcer disease and recurrent bleeding ulcers. Although aetiologies may differ from adults, it can be assumed that recurrence of H. pylori-related PUD can be prevented in children by eradication of the infection. Prospective studies of children with documented eradication of H. pylori infection have shown that the vast majority do not become re-infected over time.24
Repeating endoscopy purely to confirm H. pylori eradication cannot be justified as there are several validated non-invasive methods to confirm eradication. Stool antigen tests are convenient validated tests for H. pylori detection. Both polyclonal and monoclonal assays have been developed and tested in children. Polyclonal-based tests are slightly less reliable than UBTs.25 Validation in children under 5 years, those with gastrointestinal bleeding, and those taking acid suppressive therapy is awaited. It is likely that in the near future, monoclonal stool antigen testing will be sanctioned as an alternative to the currently recommended UBT in the post-therapy setting. Commercially available stool immune-chromatography kits are also available, but inter-observer variability and equivocal results are problematic. Newer in-office rapid monoclonal stool tests have been studied in both children and adult populations, with sensitivity and specificity of 86–91% and 91–93% respectively, although to date they remain inferior to ELISA based tests.26 ,27
The UBT has been shown to have a high sensitivity and specificity in the detection of H. pylori infection. Histological severity of inflammation and bacterial density do not directly correlate with the UBT results. When performed correctly (ie, the patient should have an empty stomach prior to receiving an acidic drink eg, citric acid based drink) the UBT is highly accurate in detecting the presence of H. pylori infection. Lower specificity has been reported in children less than 6 years. UBT in children must be interpreted qualitatively and cautiously, and consistency in UBT protocols and analyses are imperative. UBT results have potential limitations. Failure to locally validate UBTs, not using citric acid pre-treatment and the use of lower doses of 13C–urea reduce the reliability of results. Their accuracy remains questionable in young toddlers given the current lack of sufficient high quality data, although modifying analysis protocol may improve the false positive rate in children under the age of 6 years.28 Elitsur et al reported a UBT sensitivity of 98% and specificity of 96% in 176 children who received 75mg of 13C-urea plus citric acid and were tested before and 15 min after the urea administration. In those children aged 2–5 years, the urea hydrolysis rate was more sensitive than, but equally specific to, the uncorrected DOB.29
A multicenter trial in 2000 demonstrated that the DOB cut off value varied with changes in 13C—urea dose, type of test meal, and time of breath collection. Test meals that contain citric acid may improve the sensitivity of the test in children.30 A study by Kindermann et al31 of 1499 German children confirmed UBT utility in children over 6 years of age, with 149 cases being validated histologically. The positive predictive value and negative predictive value (NPV) for children over 6 years of age were 98% and 100%, but for children under the age of 6 years, they were 69% and 100%, respectively. Koletzko and Feydt-Schmidt have demonstrated a significant inverse relationship between DOB values and age in both infected and non-infected children.19 Kindermann's analysis demonstrates a false-positive rate of about 8%.31 These findings support the concept that the DOB cut-off value needs to be calculated by the receiver operating characteristic (ROC) curve for each protocol in each patient population. The accuracy of non-invasive tests remains questionable in young toddlers. One difficulty posed by this patient group is the lack of sufficient numbers of study patients.32
It is recommended to leave an adequate interval before re-testing for H. pylori. The recent ESPGHAN/NASPGHAN guidelines recommend waiting at least 2 weeks after stopping PPI therapy and at least 4 weeks after stopping antibiotics to perform non-invasive tests.7 Performing these tests too early may lead to false positive results.
In summary, non-invasive tests with either 13C UBT or stool antigen tests are reliable, validated methods to confirm H. pylori eradication in children and repeat endoscopy for this purpose is not necessary.
-
Clinical message:
-
Eradication of infection is recommended in children with H. pylori infection and peptic ulcer disease.
-
The H. pylori stool antigen test and 13C-UBT are reliable non-invasive tools to confirm H. pylori eradication.
-
Allow a minimum of 2 weeks after discontinuing proton pump inhibitor (PPI) therapy and 4 weeks after discontinuing antibiotics before non-invasive testing of H. pylori eradication.
Is there any role for serology-based non-invasive testing of H. pylori in children?
Serological tests of H. pylori exposure cannot be justified for clinical use, on either clinical or economic grounds, because of their inherent inaccuracy and misinterpretation. They are widely available, convenient and relatively cheap but should be restricted to use in epidemiological studies. H. pylori infection induces both cellular and humoral immune responses, resulting in an early increase in specific IgM and a later and persistent increase in specific IgA and IgG.33 Serologic assays cannot be used alone to diagnose H. pylori infection or to monitor for eradication of the organism. They have widely variable sensitivity and specificity for detection of antibodies (IgG or IgA) against H pylori in children.34 IgA based tests detect only 20–50% of H. pylori infected patients.34 Tests based on the detection of specific anti-H. pylori IgG antibodies in the serum offer a better sensitivity than IgA based tests but cannot distinguish active from past infection.
Office-based serology tests, although technically simple to perform and attractively convenient, are also not recommended for diagnosis, with one study reporting a 33% false positive rate in a primary care setting.35 Results with salivary and urine antibody tests have also been disappointing.9 ,36
Validation of any future, novel non-invasive methods in children must be interpreted in the context of appropriate comparison with an acceptable paediatric reference standard—otherwise they should not be used in clinical practice.37 Therefore, there is no role for serology based non-invasive testing of H. pylori in children. Tests based on the detection of antibodies against H. pylori in serum, urine and saliva should not be used in clinical practice.
Topics for future research
Developing accurate, clinically meaningful non-invasive tests for H. pylori infection and disease in children remains a goal of current research activities. Applied molecular technologies have recently been used to detect H. pylori in stool. PCR analysis is a highly sensitive technique that can be used to detect the presence of H. pylori in bodily fluids, tissues, stool and the environment. Although PCR is technically challenging and neither inexpensive nor widely available, it has the potential to compliment or even replace routine culture methods for routine clinical purposes.38 Whereas faecal bi-probe real-time PCR assays in adults have shown excellent results, results suggest a reasonable specificity but a poor sensitivity in children.39 ,40 H. pylori strains expressing certain genes and virulence factors may provoke more severe gastric mucosal pathology than others. Knowing such a bacterial profile may be useful for H. pylori disease risk prediction. Determination of in situ expression of bacterial virulence factors such as iceA, cagA, VacA, EPIYA motifs, outer membrane protein and adhesion gene expression may be useful in creating such a profile, although their implications for paediatric H pylori disease may be distinct from those of adults. Using recombinant protein technology to isolate such potential antigens could provide new perspectives for developing novel immunodiagnostic reagents and complement traditional isolation and in vitro testing of single-colony isolates.41 Recent interest has also been generated in the identification and validation of a panel of biomarkers of H. pylori infection, including pepsinogen-I and –II and gastrin-17. Although data are promising, adult studies have failed to produce convincing results to advocate its routine use to date.27 Population-based H. pylori eradication strategies have been proposed to reduce gastric cancer and PUD, but the widespread use of antibiotics involved may further increase the antibiotic resistance of H. pylori or other gastrointestinal flora.42 The population prevalence of H. pylori infection has steadily declined in recent decades, in tandem with a converse rise in atopic and autoimmune diseases in particular. While the underlying reasons are multifactorial, it is tempting to speculate on links between these phenomena. The potential population benefits of wide-scale population eradication must therefore be carefully evaluated against all potential sequelae.
Summary
The current reference standard for investigating H pylori associated disease in children remains upper intestinal endoscopy and biopsies for histology and culture or RUT. Non-invasive tests should be used to confirm H. pylori eradication following treatment. Currently there is insufficient evidence to recommend them over invasive tests in symptomatic children, because they cannot be used reliably in children to diagnose or distinguish H. pylori—associated diseases from conditions that are not H. pylori related. Recent evidence-based guidelines recommend treatment in children with confirmed H. pylori–related diseases.7 However, with further knowledge of the measurable health risks for H. pylori–infected children, or with the availability of vaccination or future treatment options, the risk-benefit relationship and recommendations regarding non-invasive testing may change.
Clinical bottom line
-
The population prevalence of H. pylori infection is declining
-
The majority of symptomatic children infected with H. pylori do not have peptic ulcer disease. The purpose of investigation remains to determine the cause of symptoms in children, rather than to determine H. pylori infection status alone
-
Empiric H. pylori eradication treatment in children because of a positive non-invasive test alone is not clinically appropriate.
-
Invasive tests are indicated for investigating causes of symptoms; validated non-invasive tests for children (UBT, monoclonal stool antigen test) are indicated for confirming H. pylori eradication post treatment.
-
Non-invasive test methodology should be rigorous, standardised and locally validated for use in the various paediatric age groups. Post-treatment non-invasive testing to confirm eradication should be deferred until at least 2 months after medical therapy is completed.
Quiz
QUESTION 1
What is the current reference standard investigation for diagnosing H. pylori?
-
Urea breath test
-
Serologic tests
-
Upper GI endoscopy
-
Upper GI endoscopy and biopsy
-
Stool antigen test
QUESTION 2
What is the main function of non-invasive testing in the diagnosis of H. pylori?
-
To investigate family members of a child with confirmed H. pylori
-
To diagnose H. pylori when a child presents with reflux symptoms
-
To confirm H. pylori eradication following treatment
-
Current ‘gold standard’ tests in the diagnosis of H. pylori
-
To rule out H. pylori in children with recurrent abdominal pain
QUESTION 3
The majority of children infected with H. pylori
-
Present with symptoms of reflux
-
Are prone to cyclical vomiting
-
Are asymptomatic
-
Present with loose bowel motions
-
Have an increased appetite when compared to non-infected children
QUESTION 4
Following successful treatment of H. pylori, the risk of recurrence is:
-
Low
-
Moderate
-
High
-
Never occurs
-
Always occurs
Answers to the quiz
-
QUESTION 1
-
Answer: D. Upper GI endoscopy biopsy
-
QUESTION 2
-
Answer: C. To confirm H. pylori eradication following treatment
-
QUESTION 3
-
Answer: C. Are asymptomatic
-
QUESTION 4
-
Answer: A. Low
Acknowledgments
Dr Michael McDermott kindly provided the pathology images used in this article.
Appendix
1. Search strategies
The literature review for this manuscript included both paediatric and adult data, where paediatric data were scarce. Electronic searches using PUBMED, Embase and CINAHL databases for all relevant articles published prior to January 2012 were used initially, and further updated in June 2012. Relevant clinical guidelines, systematic reviews, clinical trials, cohort studies, case-control studies, diagnostic studies and case series were retrieved.
References
Footnotes
-
Competing interests None.
-
Provenance and peer review Not commissioned; externally peer reviewed.